Figure: Illustration of mosaicism in eyes.
Recent breakthroughs in genetics research may have uncovered new genes underlying common psychiatric disorders. Schizophrenia and bipolar disorder affect more than 64 million people around the world. These disorders are strongly influenced by genetics. No one gene, however, determines one’s risk of developing schizophrenia or bipolar disorder. Rather, it is likely that a host of genes contribute to risk. Using artificial intelligence, researchers at Stanford University now have uncovered complex variants throughout the human genome that may contribute to these psychiatric disorders. This new study suggests that mutations that occur after fertilization, such as genetic mosaicism, may be responsible for a number of psychiatric disorders including bipolar disorder and schizophrenia.
Think of a genome as a living book with instructions for every cell in the body. Our genes are the chapters. We have approximately 20,0000 genes that provide instructions for making proteins, the building blocks of life. The vast majority of our genes, however, are non-coding, meaning that they do not provide instructions for proteins. Nonetheless, these genes play an important role in genetics and regulating cell function.
Genetic variants, or spelling changes, in either a coding or non-coding region can interfere with how the cell translates specific instructions. A small typo may have little to no effect on how the book is read. However, larger spelling changes can lead to the deletion of a sentence or even a whole chapter. Without the correct instructions to produce specific proteins, these spelling changes can contribute to disorders that impact different aspects of our body.
Our genes are a combination of the DNA we inherit from our parents. We have two copies of each gene, one from mom and the other from dad. These randomly assorted gene pairs determine traits like hair texture, eye color, and even some health risks. Some traits are dominant, meaning that only one copy of the variant is needed for expression. Others are recessive and only show up if both copies are the same. This is referred to as Mendelian inheritance, named after Dr. Gregor Mende’s initial observations of how genes are passed down in pea plants.
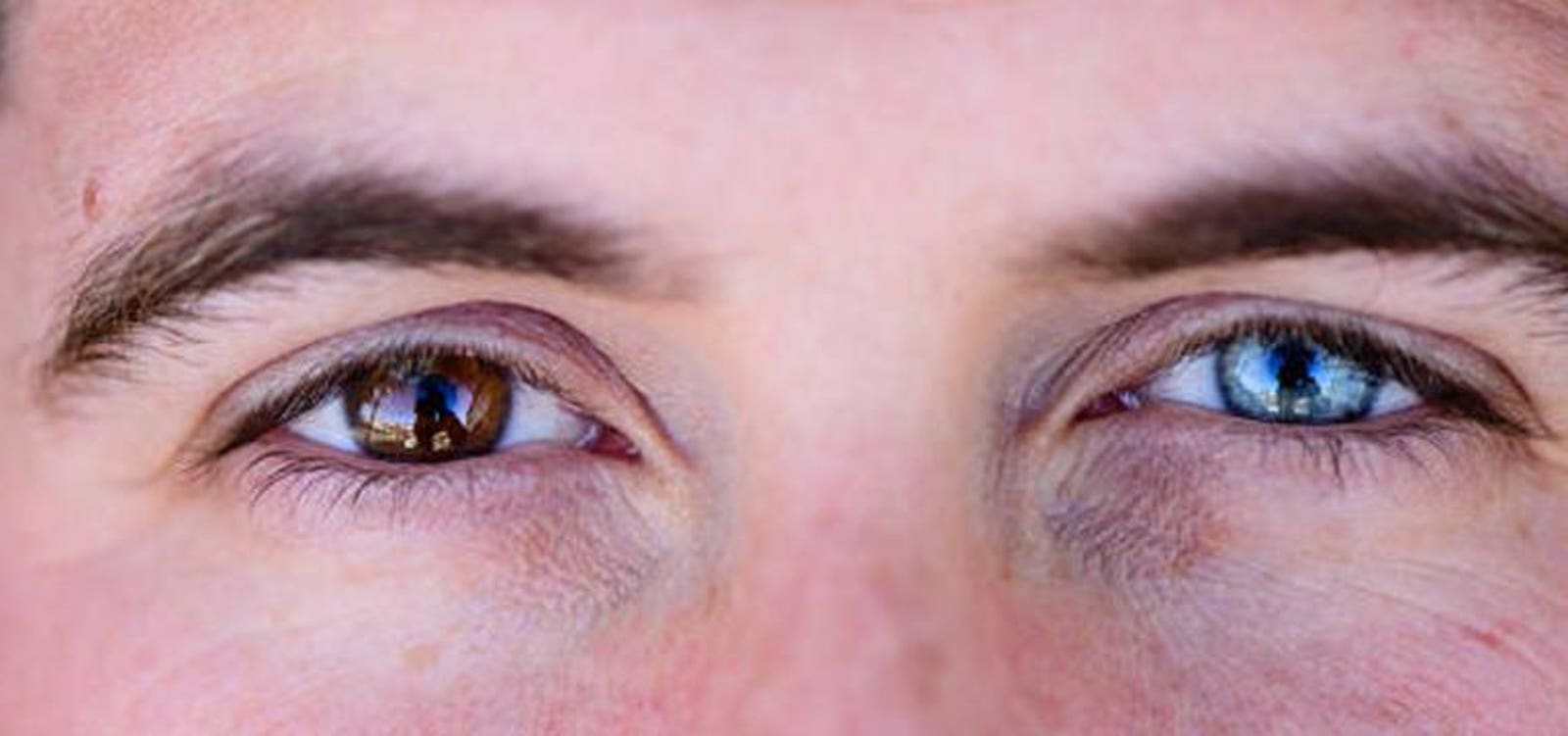
In the earliest stages of life, DNA undergoes multiple rounds of replication. Trillions of cell divisions occur, during which one cell splits into two identical daughter cells. DNA replication, however, is prone to mistakes. Each time a cell divides, tiny spelling errors are produced in the genome. Rapid replication during the first trimester of pregnancy, therefore, can introduce a host of genetic changes not seen in mom or dad. This is known as genetic mosaicism, where two or more genetically distinct cell populations are expressed in the body. Mosaicism can appear as two different color eyes, or alternating patterns of skin as shown below. A number of conditions have also been associated with mosaicism such as developmental delays, autism, epilepsy, and some cancers. We all have some degree of genetic mosaicism in our bodies. This is why identical twins can have different fingerprints.
Figure: Depiction of skin mosaicism.
Genetic variants can also be acquired throughout an individual’s lifespan that further change our genome’s mosaic. Changes in DNA may arise from exposure to chemicals or radiation, or from infections such as hepatitis B and C that corrupt the genetic material in a host cell. Other variants are acquired randomly. DNA may develop errors during replication and other normal cell functions. This damage is exacerbated by inflammation, aging, and lifestyle choices like smoking and poor diet. Pinpointing which variants contribute to certain disorders, therefore, can sometimes be a very complex process.
Whole genome sequencing (WGS) can help identify small changes in DNA. This genetic test maps an individual’s entire genome using samples collected from blood or check swabs. Whole genome sequencing extracts the exact sequences that comprise each chapter of our DNA. The extracted sequences are then compared to reference genes from a typical human genome. Any difference between an individual’s genome and the reference genome reveals a potential variant that could be associated with a disorder.
Alexander Urban, senior author of this study and Associate Professor at Stanford, describes, “Looking for only simple variations is like proofreading a book manuscript and searching exclusively for typos that change single letters. You are overlooking words that are scrambled or duplicated, or in the wrong order—you might even miss that half a chapter is gone.” Certain disorders, in fact, may be linked to long, complex spelling changes in an individual’s genes. It is made even more complicated by the fact that variants across several genes may overlap with more than one disorder.
Many psychiatric disorders are influenced by multiple changes across similar genes. Bipolar disorders and schizophrenia are prime examples of the complexity of the human genome. Hundreds of genetic variants have been identified that contribute to risk. Many of these genes are linked to brain development, immune system regulation, and neuron signaling pathways. The AKAP11 gene, in particular, has been found to be a strong risk factor for bipolar disorder, though recent studies in mice suggest that this gene may also be implicated in schizophrenia. Understanding how spelling changes in this gene interact with other high-risk variants may help to decipher what induces the onset of psychiatric symptoms.
In their study, Zhou et. al compared the genomes of over 4,000 individuals around the world. Their entire DNA sequence was extracted using whole genome sequencing. The data was then uploaded into an AI algorithm trained to recognize dozens of genomes across diverse ancestry. This approach allowed researchers to match large, complex gene variants with specific health conditions.
The study specifically recruited individuals with known bipolar disorder or schizophrenia diagnoses and compared them to healthy controls. This type of approach is known as a genome-wide association study (GWAS). Genome-wide association studies compare the genes of individuals with a particular disease to a large cohort of controls. While this approach can tell us where variants are located, this information is often not precise. For instance, it may tell us that the book contains spelling changes on pages 122, 296, and 731, but not what type of errors are involved. The AI algorithm developed by Zhou et. al adds more specificity. It highlights the changed word or sentence and reports whether it has been scrambled, duplicated, or deleted.
With more than 85% accuracy, the AI tool identified more than 8,000 complex variants. Many of these spelling changes were found in regions of the genome that provide instructions for brain function. To determine if these variants could be linked to psychiatric disorders, they extracted DNA from brain tissue samples of individuals affected by schizophrenia or bipolar disorder. The complex variants that they identified seemed to overlap with single variants found in other genome-wide association studies of these disorders. For instance, one complex variant that they found correlated with schizophrenia and bipolar disorder was the length of 4,700 base pairs, the basic unit of DNA. In the book analogy, base pairs are like the words in the book.
New innovations in genetic research are deepening our understanding of the human genome. By analyzing vast amounts of genetic data, AI technology is uncovering intricate relationships between large variants and certain psychiatric disorders. This not only enhances our understanding of the genetic basis of these disorders but also paves the way for personalized medicine. As we continue to uncover more of the human genome, future studies may reveal deeper insights into the genetic underpinnings of an array of disorders.






